
Many diseases caused by viruses (or bacteria) do not immediately harm their host. Often, the relationship between pathogen turnover, immune activation and disease progression remains obscure. Theoretical models allow to determine key quantities such as the daily viral turnover in vivo, infected cell death rates and related cytopathicity, or the number of secondary infected cells per primary infected cell. This information is crucial for the monitoring of patients, quantitating of the efficacy of antiviral interventions, and the design of novel therapeutic interventions.
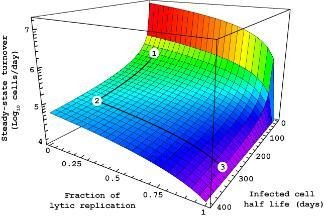
Infected host cell turnover en route to
PTLD in an Epstein-Barr virus infected
transplant patient. Depicted is the trade-
off between the fraction of lytic versus
episomal replication and the half life of
infected host cells.
For details, please consult: Funk et al.,
2007, The Lancet Infectious Diseases.
I'm particularly interested in metapopulation dynamics. Metapopulation theory has been successfully applied in conservation biology to explore how long-term survival of species depends on a shifting balance between local extinction and repopulation events in spatially structured landscapes. Interactions between pathogens and the immune system within the body of infected hosts show parallels to metapopulation ecology. The ultimate goal of interventions, however, is exactly opposite: eradication of a pathogen instead of conservation of a rare species. Spatially structured models represent an important step forward to understand what happens when the assumption of well-mixed pathogen populations within a host is relaxed.
